Surface characterization of nanocrystalline strontium titanate
SrTiO3 (STO) single crystals have been extensively studied as a model for mixed-metal oxide catalyst supports. However, high surface area (nanoscale) materials are essential for real catalytic applications, but relatively little is known about the surface atomic structure and chemistry of nanocrystalline STO. While recent studies have demonstrated the effects of support material and crystallographic orientation, current efforts aim to further elucidate the role of the support at the molecular level by examining the surface functional groups present on STO nanocuboids prepared by hydrothermal synthesis. The focus here is on the surface hydroxyl groups which serve as reactive sites for the atomic layer deposition (ALD) of catalytic metal oxide species. The variation in the density and dispersion of hydroxyl groups on a support surface can dictate the possible bonding configurations for the deposited catalytic material, so control of hydroxyl group chemistry may allow for control of the active sites to enable catalysis of essential industrial reactions. This research seeks to obtain a better understanding of how the support surface chemistry influences ALD, and how it can be controlled to optimize the activity, selectivity, and stability of the catalyst..
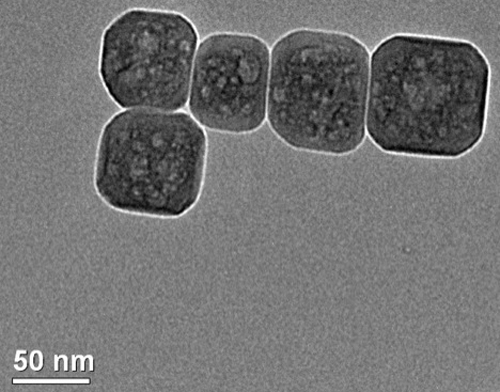 Figure 1: STO nanocuboids prepared by microwave hydrothermal synthesis
Figure 1: STO nanocuboids prepared by microwave hydrothermal synthesis
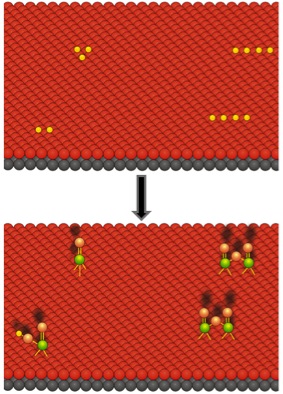 Figure 2: Hydroxyl dispersion on the nanocuboid support surface can dictate the atomic layer deposition of catalytic oxides, resulting in the presence of different active sites (yellow dots represent hydroxyl groups)
Figure 2: Hydroxyl dispersion on the nanocuboid support surface can dictate the atomic layer deposition of catalytic oxides, resulting in the presence of different active sites (yellow dots represent hydroxyl groups)
Grant: ICEP

