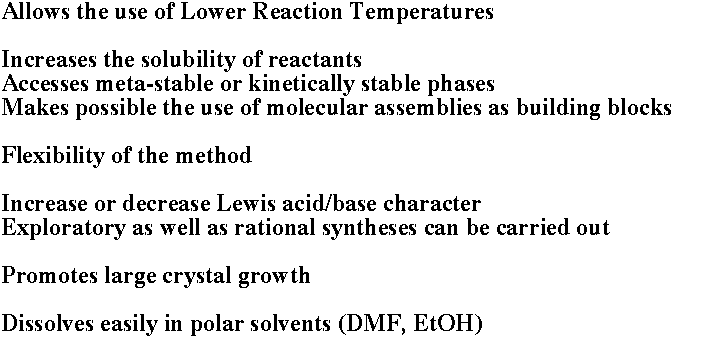
The application of salt fluxes in the synthesis of new solid state compounds has witnessed significant development in the last few years. Particularly important has been the emergence of the molten polychalcogenide flux methodology in the exploratory synthesis of complex chalcogenides. This approach to new chalcogenides has been developed and brought to the main stream of inorganic chemistry methodology mainly in our laboratories.
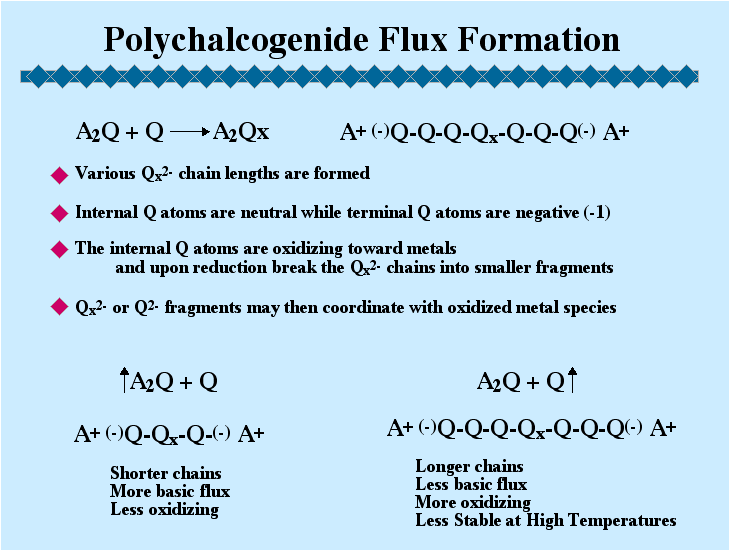
This method of synthesis and exploration has simplified access to low and intermediate temperatures (160-600 oC) and contributed to the discovery of some very interesting materials. In many cases the compounds stabilized under polychalcogenide flux conditions are only kinetically stable and cannot be synthesized at higher temperatures. Thermodynamic influences, however, are not entirely avoided by this approach. (Kanatzidis M. G.; Sutorik A. C. "The Application of Polychalcogenide Salts to the Exploratory Synthesis of Solid State Multinary Chalcogenides at Intermediate Temperatures" Progr. Inorg. Chem., 1995, 43, 151-265.)
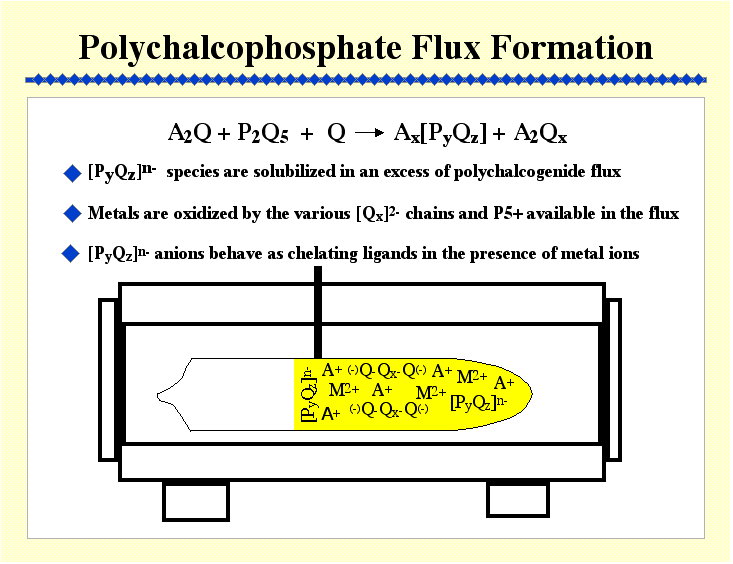
Lower temperatures, coupled with the presence of a flux, also make possible the use of molecular assemblies as building blocks for incorporation into solid state structures, and consequently the construction of complex multinary solids. The latter two aspects are the subject of our current attention with the main emphasis being the generation, control, and understanding of the reactivity of flux-assembled anions
These include:
Thiophosphates ([PxSy]n- anions)
Selenophosphates
Thiogermanates, thiostannates ([GeySz]n- and [SnySz]n- )
Selnogermanates and selenostannates ([GeySez]n-
and [SnySez]n- )
![]()